Case Experience and Clinical Data in Over 500 Cases Using a Hybrid Biomaterial for Hernia Repair
The advantages and disadvantages of using synthetic, biologic and biosynthetic material for hernia repair and abdominal wall reconstruction procedures remain a subject of debate.1 Selecting the most appropriate material must take into account key patient characteristics, strength, and durability, as well as the risk for complications and recurrence, in achieving long-term outcomes.2,3 Although biologic and bioabsorbable devices may provide a lower risk for infection and other complications associated with the use of a foreign body in tissue reinforcement, in the past, the loss of strength and durability were disadvantages.4 Although no single surgical mesh material is ideal for all patients, there has been incremental progress from an innovative materials company that has developed a differentiated hybrid biomaterial for hernia repair and abdominal wall reconstruction that is biocompatible while providing the strength and durability of synthetics and potentially minimizing the risk for complications, as perceived with biologics.
“We are seeing several advances over the last 5 to 10 years in the technology of hernia repair that are allowing better incorporation of mesh into the abdominal wall,” said Cheguevara Afaneh, MD, an assistant professor of surgery at NewYork-Presbyterian Hospital, in New York, New York. “We have moved to the concept of a partially absorbable mesh in our search for more reliable outcomes in situations where strength is a concern.”
Biosynthetic material leverages the benefits of biologic and synthetic materials, facilitating revascularization while minimizing risk for complications. More recently, the availability of a new hybrid biomaterial, GORE® SYNECOR Biomaterial products, presents an innovative alternative that combines the advantages of synthetic and bioabsorbable materials for hernia repair and abdominal wall reconstruction to provide long-term strength with rapid tissue ingrowth and vascularization through a single-stage repair, which is preferable for complex cases and high-risk patients with multiple comorbidities. Clinical experience with GORE® SYNECOR Biomaterial products for hernia repair and other abdominal and thoracic wall reinforcement exceeds 3 years. The results show few complications,5 and several clinical reviews are currently in progress. Results showed low recurrence rates following use of GORE® SYNECOR Biomaterial products.6
Clinical experience with GORE® SYNECOR Biomaterial products confirms the advantages of this hybrid biomaterial, largely circumventing the key disadvantages of synthetic, biologic, and biosynthetic devices while preserving their positive attributes. In particular, a high degree of material strength is preserved while the foreign body complication rate is minimized. The outcomes of 2 large independent case series representing over 500 clinical cases were presented at the 19th Annual Minimally Invasive Surgery Symposium. The results further validated durable strength, tissue ingrowth, and low recurrence rates with GORE® SYNECOR Biomaterial products, supporting their use in complex and high-risk cases. The outcomes were viewed by the surgeon presenters as providing an important step forward in achieving quality long-term outcomes for patients.
Current Landscape: Mesh and Biomaterials
With more than 70 mesh devices for hernia repair currently on the market,2 it is evident that the opposing risks and root causes for hernia repair failure are not easily reconciled. The correct choice of reinforcement material is not the same across cases due to the variables that increase risk for an adverse outcome, as well as the construction of the device material. Reherniation is often the primary risk for a patient with a prior history of abdominal surgery, comorbidities such as obesity, or a large hernia defect. Infection, inflammation due to the device, or chronic pain may be considered more important in patients with comorbidities or prior recurrences. The key components and chemistry of mesh materials and biomaterials available are not well understood, leading to confusion in materials and selection. Complications and other considerations, such as cost, require the choice to be individualized by case characteristics.
Synthetic and Biologic Mesh
The 3 most popular synthetic permanent materials are polypropylene, polyester, and polytetrafluoroethylene (PTFE). Because biologic mesh material derives from decellularized human or animal tissue, it can potentially facilitate revascularization and ingrowth with the host tissue, potentially reducing the risk for inducing mesh-related complications.7 The use of biologic devices, however, is hindered by limited long-term outcomes, potential degradation, and high costs.8 Fully synthetic absorbable devices with varying absorption periods have been constructed to help meet different patient needs (eg, PGA/TMC, PGA/PLA, P4HB, and polyglactin 910).
Biosynthetic Mesh
Biosynthetic mesh combines the attributes of synthetic and biologic mesh materials to create a new material that can be used in complicated repairs, while minimizing risks for complications. Among the currently available biosynthetics, GORE® BIO-A® Tissue Reinforcement enables tissue generation while maintaining a low inflammatory response and minimizing risk for long-term mesh-related complications. Dr Afaneh discussed data comparing several biologic meshes with GORE® BIO-A® Tissue Reinforcement for relative upregulation of the proinflammatory interleukin-1beta (IL-1beta) cytokine and reported no substantial change in cytokine level with GORE® BIO-A® Tissue Reinforcement, which was matched by one but not all of the biologic meshes tested.9 “The biologics would be expected to be the least inflammatory of the meshes that are out there, but GORE BIO-A® Tissue Reinforcement actually behaved better than some biologics,” he said.
Made of a unique 3-dimensional (3D) biosynthetic web scaffold consisting of 67% polyglycolic acid and 33% trimethylene carbonate (Gore proprietary PGA:TMC Web Technology), the GORE® BIO-A® Tissue Reinforcement provides a 100% synthetic, bioabsorbable tissue scaffold that is engineered for uniformity, consistency, and biocompatibility to ensure cellular ingrowth and vascularization.10,11 Within 6 to 7 months of placement, GORE® BIO-A® Tissue Reinforcement is broken down primarily by hydrolysis.11 “GORE® BIO-A® Tissue Reinforcement has been around for about a decade and has been widely used,” Dr Afaneh said. “It is a tried-and-true material that has not been associated with esophageal erosions when used in hiatal hernia repair.” The 3D construction of BIO-A® Tissue Reinforcement is credited with rapid neovascularization and rapid ingrowth of an organized collagen structure that produces high-quality tissue. These advantages were demonstrated in a comparative study with other biologics, including ETHICON FLEXHD® Acellular Hydrated Dermis and ALLERGAN STRATTICE® Reconstructive Tissue Matrix, in which GORE® BIO-A® Tissue Reinforcement was associated with a greater extent of cellular ingrowth and vascularization.12
GORE® SYNECOR Biomaterial Products: The Hybrid Alternative
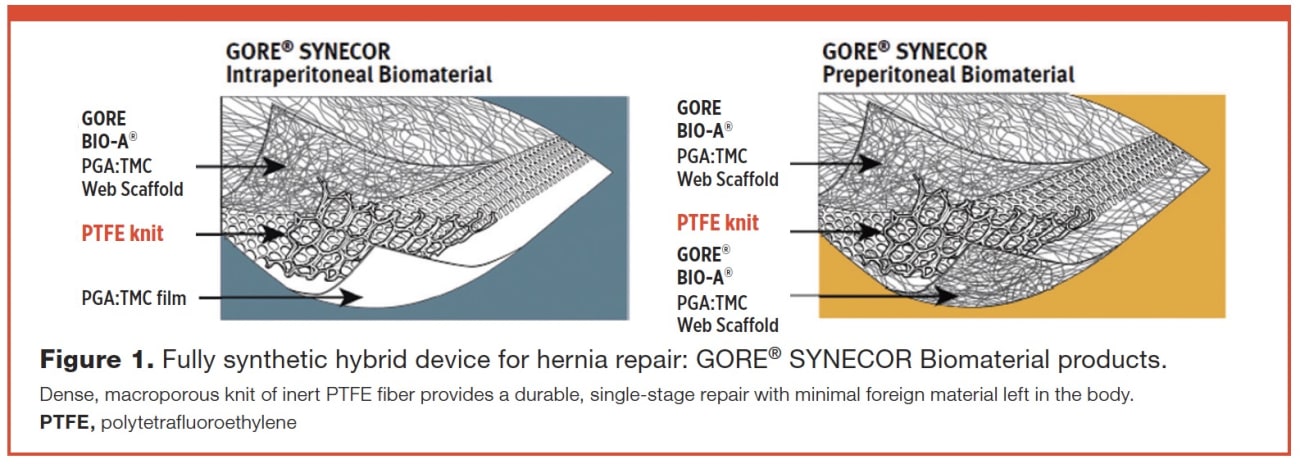
GORE® SYNECOR Biomaterial products combine the tissuebuilding scaffold of Gore proprietary PGA:TMC Web Technology with a layer of dense monofilament polytetrafluoroethylene (PTFE) knit fibers to facilitate rapid cell infiltration and vascularity, while optimizing strength for a durable repair.13 The construction of the PTFE knit was shown to have minimal bacterial adherence.14 The solid fiber of this macroporous PTFE knit has a diameter similar to lightweight polypropylene, but has a high strength as measured by burst strength for a permanent repair, while leaving minimal material behind in the body long term (Figure 1).
“There is a big difference in the hybrid technology of [GORE® SYNECOR Biomaterial] compared to the construction of the older technology of microporous sheets and current polypropylene and polyester knits,” said Carl Doerhoff, MD, of Surgicare West Trauma Clinic & Center of Orthopedic Excellence in Jefferson City, Missouri.
In experimental studies using fluorescence, Dr Afaneh noted that when the bacterial adherence was studied with confocal imaging, the PTFE knit in GORE® SYNECOR Biomaterial products showed that no bacteria were located within the PTFE knit fibers and overall fewer bacteria were located in the PTFE knit than other materials examined.13
With both GORE® SYNECOR Biomaterial products,“blood vessel growth within the Gore 3D PGA:TMC web scaffold is already observed within 7 days,” Dr Afaneh said. “With progressive neovascularization and tissue integration into the device, this may increase the durability of the surgical repair long term.” This is a unique attribute of hybrid construction. Dr Afaneh cited a study that demonstrated an almost doubled tensile strength at 6 months compared with 2 weeks, even though most or all of the BIO-A® Tissue Reinforcement would be expected to have been absorbed by that time.15 “Patients like to hear that part of the mesh is resorbed and is replaced with their own collagen,” he said. He indicated that native tissue integration with the biosynthetic layer incorporates the device into the abdominal wall at the repair site.
According to Dr Doerhoff, “One of the most important advantages of GORE® SYNECOR Biomaterial products is that it has a very high burst strength.”
The versatility of GORE® SYNECOR Biomaterial products can be applied to a variety of surgical approaches, including transverse abdominis muscle release (TAR), high-risk ventral hernia repair, and other component separation techniques.13 Working with GORE® SYNECOR Biomaterial products primarily in robotic repairs, Dr Afaneh reported that a substantial proportion of cases in his tertiary setting are challenging referrals. GORE® SYNECOR Biomaterial enables the use of single-stage durable repair for challenging cases, which may reduce overall costs associated with the procedure.13 Dr Doerhoff also emphasized the ease of handling characteristics and versatility of GORE® SYNECOR Biomaterial products. “It can be easily trimmed intracorporeally, which is an advantage when tailoring mesh in the lower abdomen and groin areas,” he said.
Clinical Experience in Over 500 Cases

For more than 3 years, Dr Doerhoff has used GORE® SYNECOR Biomaterial products in over 250 abdominal wall reconstructions. Mesh has been placed by open, laparoscopic, and robotic techniques. Dr Doerhoff reported only 2 recurrences and each was unrelated to the mesh itself. He believed that each recurrence occurred because a larger piece of mesh should have been used and more fixation would have been appropriate. Both recurrences were repaired using a larger piece of GORE® SYNECOR Biomaterial and more fixation (Table 1)
Dr Doerhoff has videos of implanted GORE® SYNECOR Biomaterial in patients who required other non-hernia procedures, as well as second, third, and fourth looks at GORE® SYNECOR Biomaterial products demonstrating minimal contraction at the periphery of one mesh and no mesh failure (Figure 2).

Although Dr Afaneh is currently following over 250 abdominal wall repairs performed using GORE® SYNECOR Biomaterial products, he restricted his outcomes report to the first 91 cases undergoing ventral or incisional hernia repair who had long-term follow-up (Table 2). In a complicated patient population, 77% had undergone previous abdominal surgery and 38% were recurrent hernia repairs. Of these patients, 60% had an American Society of Anesthesiologists physical status score of III or higher, signifying the presence of significant systemic disease. In this series, the case mix included intraperitoneal onlay mesh, TAR, preperitoneal, retromuscular repairs, and some patients had concomitant diastasis repairs.
With a substantial proportion of patients referred from the liver and kidney transplant service at his center, Dr Afaneh noted that 21% of patients in this series were immunosuppressed at the time of surgery. The average hernia size was 7 cm. In 11% of cases, the hernia was incarcerated or strangulated. After a mean follow-up time of 7 months, there was a 1% rate of intraoperative morbidity, a 13% rate of postoperative morbidity, and a 5% rate of recurrence. “Given that a substantial proportion of patients had previous abdominal wall surgery and a high risk for complications, these results are quite promising. Many of the recurrences we did see occurred on the outskirts of the mesh rather than centrally,” he said, crediting these encouraging results to features of the hybrid design and the high strength profile. In particular, Dr Afaneh emphasized
the benefit of the low inflammatory response to the GORE® SYNECOR Biomaterial products and, in his opinion, this surgical repair gains, rather than loses, strength over time through tissue integration into the mesh.

Conclusion
Although the debate on the optimal mesh material continues, progress has been made in developing innovative materials and devices that facilitate a more individualized approach to hernia repair and other abdominal wall reinforcement procedures. Ultimately, the value analysis of selecting a mesh will need to take into account risks for recurrence and infection, as well as patient satisfaction and acquisition costs. The availability of GORE® SYNECOR Biomaterial products as unique hybrid devices provides a strong and durable alternative to lightweight synthetic and biologic meshes, particularly for challenging abdominal wall hernia repairs, in which the risks and the total costs of care are greater.
References
1. Vorst AL, Kaoutzanis C, Carbonell AM, et al. Evolution and advances in laparoscopic ventral and incisional hernia repair. World J Gastrointest Surg. 2015;7(11):293-305.
2. Baylón K, Rodriguez-Camarillo P, Elias-Zuniga A, et al. Past, present and future of surgical meshes: a review. Membranes (Basel). 2017;7(3). pii: E47.
3. Majumder A, Winder JS, Wen Y, et al. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery. 2016;160(4):828-838.
4. Kamarajah SK, Chapman SJ, Glasbey J, et al. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open. 2018;2(6):371-380.
5. Grimsley L, Forman B, Carbonell A, et al. Evaluating the real world use of a new hybrid hernia mesh. Hernia. 2018;22(suppl 1):S178. Abstract P-1316.
6. Bilezikian J, Israel I, Appleby P, et al. An evaluation of ventral hernia repair with a new prosthetic mesh. Hernia. 2019;23(suppl 1):S97-S98. Abstract P-1259.
7. Huntington CR, Cox TC, Blair LJ, et al. Biologic mesh in ventral hernia repair: outcomes, recurrence, and charge analysis. Surgery. 2016;160(6):1517-1527.
8. Schneeberger S, Phillips S, Huang LC, et al. Cost-utility analysis of biologic and biosynthetic mesh in ventral hernia repair: when are they worth it? J Am Coll Surg. 2019;228(1):66-71.
9. Orenstein SB, Qiao Y, Kaur M, et al. Human monocyte activation by biologic and biodegradable meshes in vitro. Surg Endosc. 2010;24(4):805-811.
10. W. L. Gore & Associates, Inc. GORE® BIO-A® Tissue Reinforcement. www.goremedical.com/products/bioatissue. Accessed September 9, 2019.
11. W. L. Gore & Associates, Inc. Matrix for Tissue Generation and Healing. July 2011. www.vingmed.se/wp-content/uploads/2013/10/BIO-ATissue-Reinforcement.pdf. Accessed September 9, 2019.
12. Zemlyak AY, Colavita PD, Tsirline VB, et al. Absorbable glycolic acid/trimethylene carbonate synthetic mesh demonstrates superior in-growth and collagen deposition. Presented at the Abdominal Wall Reconstruction (AWR) Meeting; June 14-16, 2012; Washington, DC. Abstract 35.
13. W. L. Gore & Associates, Inc. Advancing the Art of a Single-Stage Repair. June 2017. www.goremedical.com/resource/AV0682-EN2. Accessed September 9, 2019.
14. Clinger L. PTFE Knit Microbial Placement. Flagstaff, AZ; W.L. Gore & Associates, Inc; 2018. [Workplan]. WP110158.
15. Pascual G, Sotomayor S, Rodriguez M, et al. Repair of abdominal wall defects with biodegradeable laminar prostheses: polymeric or biological? PLoS One. 2012;7(12):e52628.
Refer to the Instructions for Use for a complete description of all warnings, precautions, and contraindications. Products listed may not be available in all markets. All other trademarks are the property of their respective owners. ALLERGAN and STRATTICE are trademarks of Allergan, Inc. ETHICON and FLEXHD are trademarks of Ethicon, Inc. GORE, BIO-A, SYNECOR, and designs are trademarks of W. L. Gore & Associates. Gore products referenced within, if any, are used within their FDA approved/cleared indications. Gore does not have knowledge of the indications and FDA approval/clearance status of non-Gore products. Gore makes no representations as to the surgical techniques, medical conditions, or other factors that may be described in the article(s). The reader is advised to contact the manufacturer for current and accurate information.
Disclosures: Drs Afaneh and Doerhoff reported that they are on the speakers bureau for W. L. Gore & Associates, Inc.
Disclaimer: This monograph is designed to be a summary of information. While it is detailed, it is not an exhaustive clinical review. McMahon Publishing, W. L. Gore & Associates, Inc., and the authors neither affirm nor deny the accuracy of the information contained herein. No liability will be assumed for the use of this monograph, and the absence of typographical errors is not guaranteed. Readers are strongly urged to consult any relevant primary literature.
Copyright © 2019 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.